“The Holy One, Blessed be He, gives the refuah (remedy) before the makka (disease),” our Jewish sages wrote.
In 1796, Edward Jenner scratched a boy’s skin and exposed him to cowpox. The boy developed a mild fever and a blister popped up. Six weeks later, Jenner injected the boy with fluid from a smallpox sore. Eureka! No signs of the deadly disease!
How did this mother of all vaccines protect 8-year-old James Phipps? After exposure to the cowpox, the boy’s immune system flooded the bloodstream with antibodies to fight the germs. When the boy was exposed to the live smallpox, the circulating antibodies knocked out the smallpox germs before they had a chance to make the boy sick. Because cowpox is similar to smallpox, the antibodies produced by cowpox were able to kill the smallpox germs.
The beauty of vaccines is that most are highly effective, very safe and inexpensive to produce on a massive scale. Adding to their appeal, one vaccine will protect a person for many years, often a lifetime.
New Frontier: Developing Vaccines to Fight Cancer
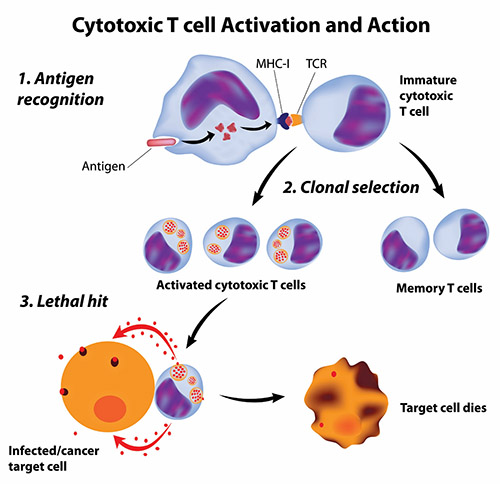
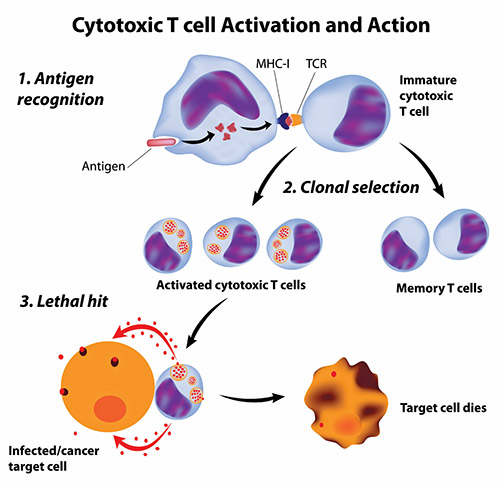
Getting the immune system to attack cancer cells is not simple. Cancer cells are not totally foreign bodies like microbes; after all, they are human cells, albeit damaged and growing out of control. However, cancer cells often have abnormal components that the immune system could view as foreign antigens (harmful substances that cause the immune system to fight back). But these antigens may not be so foreign—they may be on the cancer cells themselves—and avoid being spotted by the immune system; even when recognized as imposters, the immune system may fail to stop these “trojan cancers” from overtaking the body.
Scientists have pursued two types of cancer vaccines: vaccines to prevent cancers and, more ambitiously, vaccines to treat cancers that have already started in the body.
Preventive Cancer Vaccines Work!
Preventive cancer vaccines are like traditional vaccines: they prevent cancer from developing in healthy people. They often attack viruses that cause cancer. A notable success story is Merck’s Gardasil® 9-valent vaccine, which is effective in preventing human papillomavirus (HPV) cancers. The FDA has also approved multiple vaccines that protect against hepatitis B virus infection, which can also lead to cancer.
Treatment Cancer Vaccines: The Ultimate Challenge!
Cancer treatment vaccines attack cancers that have already secured their positions inside the body.
Producing effective treatment vaccines has proven more difficult and challenging than developing cancer preventive vaccines because cancer treatment vaccines must achieve two goals to be effective. First, like preventive vaccines, cancer treatment vaccines must stimulate specific immune responses against the correct target. Second, the immune responses must be powerful enough to overcome the barriers that cancer cells use to protect themselves from attack by killer T cells.
Save the Whale! Save the Prostate!
Whales and men need to protect their prostates to reproduce. After I heard about innovative research to develop a prostate cancer treatment vaccine, I told my urologist.
“I don’t mean to dampen your enthusiasm,” Dr. Fogelman said, “but making a vaccine that treats the patient without resorting to radical surgery, chemotherapy, radiation or other invasive measures—that’s a tall order.”
Treatment Vaccines for Prostate Cancer—A Distant Dream?
“A vaccine to treat prostate cancer is no longer a distant dream,” said Dr. Jonathan Head, CEO of OncBioMune Pharmaceuticals.
After earning his doctorate at Fordham University in 1989, a young tumor cell biologist named Jonathan Head began his quest to discover treatment vaccines for cancer. Dr. Head joined forces with Dr. Robert Elliott, a surgeon whose dream was to discover new treatments and drug therapies to cure breast cancer. Their research resulted in the awarding of a patent for one of the first autologous breast cancer vaccines in the United States. Later, based on that research, they developed a treatment vaccine for prostate cancer, which they called ProscaVax.
“ProscaVax is unique because the combination of two immune stimulants (GM-CSF and IL-2) with PSA (an antigen that is present on prostate cancer cells) results in a significant immune response to the PSA,” Dr. Head said. “This immune response attacks the prostate cancer cells in prostate cancer patients with rising PSAs in early-stage patients who had not undergone therapy, as well in previously treated patients. The vaccine’s lack of significant toxicities makes it possible to serve as primary therapy for patients in active surveillance.”
The U.S. Navy Cancer Vaccine Program funded the company’s phase 1 trial. “The interim data to date has been extremely compelling!” wrote Dr. Head in a press release (May 3, 2017). “The data demonstrates a strong safety and tolerability profile of ProscaVax. Even though it is not the primary goal of a phase 1 trial, we saw indications of efficacy with respect to slowing tumor progression and supporting an immune response to disease.”
A Costly Endeavor
Going from the lab to drug approval is a costly endeavor. Dr. Head and Dr. Elliot were zealous about their dream and dove into the ocean of investors to form OncBioMune Pharmaceuticals. Most investors are good people and help companies succeed; however, a few are sharks.
“The original investors and those who helped the company go public have been dumping their shares during the last two years, causing our stock price to be under constant pressure,” said Andrew Kucharchuk, chief financial officer of OncBioMune Pharmaceuticals, who joined after those nearly deadly deals.
“Thankfully, we found new investors who see ProscaVax’s potential and who want to see it succeed,” Kucharchuk said. “Their funding is supporting our phase 2 and 3 trials.”
Moving to the Next Phase?
In December 28, 2016, OncBioMune issued a press release stating that approximately 30 patients had been prescreened to participate in the phase 2/3 trial of ProscaVax for late-stage prostate cancer. January, February, March, April and May passed. I was worried.
Then, on June 27 (the miraculous day of Gimel Tamuz when Joshua delayed the setting of the sun), I saw an announcement in my email: “Phase 2/3 Trial in Mexico to Begin.” OncBioMune Pharmaceuticals received formal regulatory approval to begin the trial!
“We are excited to announce that we have started enrolling patients,” Kucharchuk said. “The aging population and widespread adoption of screening for prostate-specific antigens have resulted in a prostate cancer crisis in Mexico. We believe we can provide a safe and effective solution to the nation’s dilemma—without unpleasant side effects!”
“This is what we’ve been waiting for and we greatly look forward to enrolling the first patient in the trial in our bid to fill an unmet medical need in Mexico,” Kucharchuk said. “Unfortunately, the more than 300,000 Mexican men diagnosed with prostate cancer each year have limited therapeutic options and none without a high degree of risk of morbidities. We hope to change that with ProscaVax, which we believe will reproduce the strong safety and efficacy profiles that we saw in the phase 1 study at the University of California-San Diego.”
Phase 2 Trial at Harvard School of Medicine
In May 2017, OncBioMune submitted a phase 2 protocol for review by the FDA. As proposed in the protocol, the trial will enroll 120 early-stage prostate cancer patients; 40 patients will be randomly assigned to the standard active surveillance group, and 80 patients to the group receiving only ProscaVax. The comparison will help determine if ProscaVax will work as a standalone treatment.
Two renowned experts in prostate cancer, Dr. Rupal Bhatt and Dr. Glenn Bubley of the Harvard University School of Medicine, have agreed to serve as the co-principal investigators of the ProscaVax trial.
“We are optimistic that the phase 2 trial at Harvard Medical School will show ProscaVax to be safe and effective for prostate cancer patients in active surveillance,” said Dr. Head.
Men who show early signs of prostate cancer and who have not received any treatment may be eligible to enter the Harvard trial. The trial is expected to start in early 2018. Enrollment information will be listed on the Clintrials.gov website this fall.
Why Have I Written This Article?
Disclaimer and cautionary statement: I am a shareholder in OncBioMune Pharmaceuticals. As a medical writer who has worked for pharmaceutical companies since 1993, I am aware that investing in small biotech companies may involve substantial risk. Numerous factors may seriously impact the approval and launch of any new drug or vaccine. I hope this article will encourage people—who can assume the appropriate risk—to invest in struggling startup biotechs that have promising, life-changing remedies that may lead to a refuah shleimah for many people.
(Sponsored by Tzvi Jacobs)
Tzvi Jacobs is a freelance writer and author of many published articles, stories and books, including “From the Heavens to the Heart” and “Who’s Gonna Save the World? © Tzvi Jacobs Edition: July 2017.” Contact the author at [email protected] for permission to publish this article and to receive an updated Word version.